Department of Chemistry, Gakushuin University
AKIYAMA Group



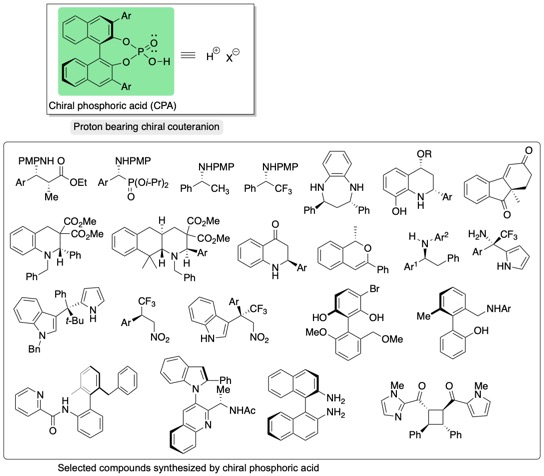
We developed chiral phosphoric acid derived from (R)-BINOL as a chiral Brønsted acid, and reported Mannich-type reaction of ketene silyl acetal with aldimines, leading to the formation of β-amino esters highly enantioselectively. Chiral phosphoric acid (CPA) functioned as chiral Brønsted acid, and is considered to be chiral proton (proton bearing chiral counteranion). Since our first report on the chiral phosphoric acid catalysis in 2004 (Angew. Chem. Int. Ed. 2004, 43, 1566-1568), chiral phosphoric acids have emerged as versatile general chiral acids. Use of transition metal catalysts could be obviated. We have reported numerous kinds of transformation using CPA. Selected chiral compounds synthesized using CPA in our group are shown below.
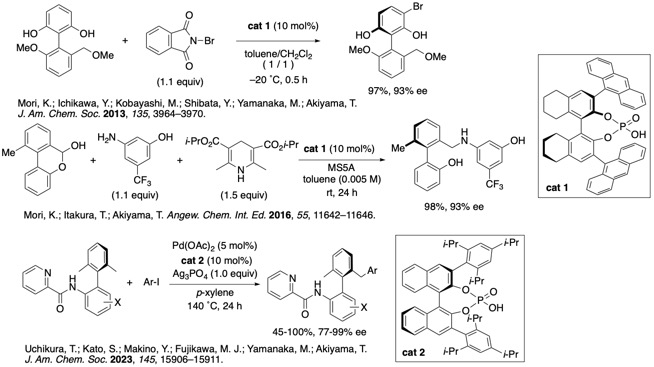
Axially chiral compounds are found in catalysts, ligands, as well as biologically active compoudns. We are interested in the construction of axially chiral compounds using CPA. We reported enantioselective bromination of Cs-symmetric biaryls using CPA by desymmetrization. Reductive amination of hemiacetal also proceeded smoothly. Dynamic kinetic resolution was achieved. We also developed an enantioselective synthesis of axially chiral biaryls by desymmetrization using C(sp3)−H activation catalyzed by chiral palladiumphosphate. This is the first report of asymmetric desymmetrization of axially chiral compounds by C(sp3)−H activation.
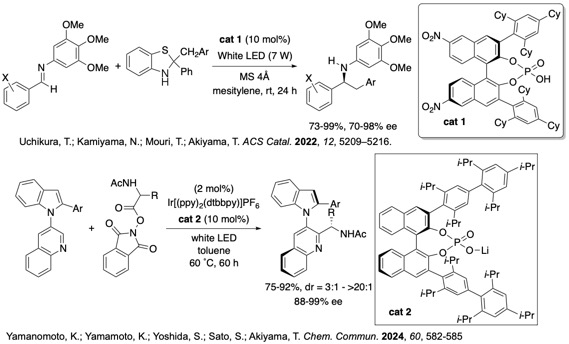
Chiral phoshoric acids can be used under photochemicall conditions as well as under thermal conditions. Benzyl group transfer reaction toward imines proceeded highly enantioselectively. Enantioselective Minisci reaction of redox-active ester with N-quinolyl indole furnished addition products with high diastereo- and enantioselectivity. Axially chiral N-quinolyl-indoles were obtained highly enantioselectively.
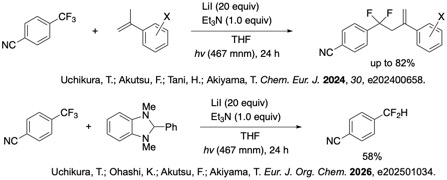
Organofluorine compounds are widely found in pharmaceuticals and agrochemicals. We have developed efficient method for the mono-selective C-H functionalization of CF3 arenes using EDA complex under photoirradiation.